




On May 25, 2022, the Shanghai Medical Products Administration released the Report on Adverse Reactions and Monitor for Cosmetic in 2021 (hereinafter referred to as “the Report”). It summarizes the data on monitoring structure, age distribution, involving products, sales channels, main reaction symptoms and body parts for adverse reaction.
Key data and indexes depicted in the Report include (as of end of 2021):
· Registration status: 952 entities from Shanghai have registered in the National Monitoring System for Adverse Reactions of Cosmetics, including 557 responsible entities, 17 regional monitoring center, 27 operating enterprises and 213 manufacturers.
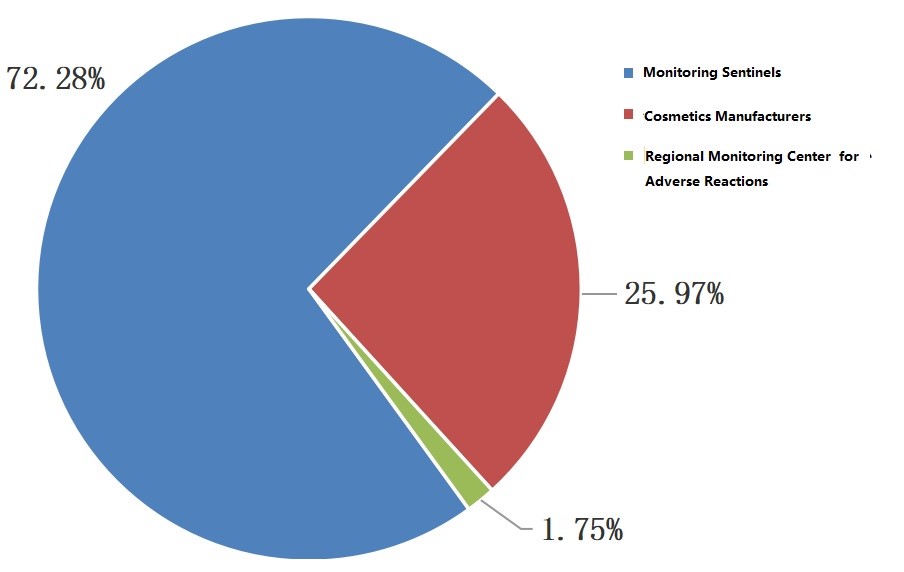
· General data: in 2021, 3027 adverse reactions/incidents were reported, and mainly came from monitoring sentinels (medical institutions). For further detail please check the diagram below.
Figure 1: Source for Reporting Adverse Reactions





 Finish the registration payment to unlock meeting access.
Finish the registration payment to unlock meeting access.